A next-generation respiratory inhaler platform designed to eliminate risk, improve reliability, and redefine inhalation therapy.
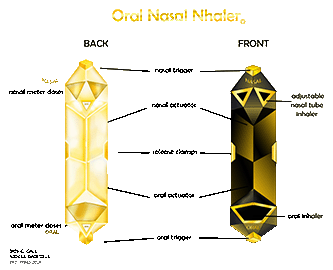
ONN (Oral Nasal Nhaler) is a patented hybrid inhaler system that delivers medication through both oral and nasal pathways in a single device. By removing the single point of failure found in traditional inhalers, ONN offers a safer, more dependable solution for respiratory patients worldwide.

150+ Global Patents
Dual Oral–Nasal Delivery
FDA 510(k) Pathway
Designed for Adults & Children
Millions of patients rely on inhalers every day to manage asthma, COPD, and other respiratory conditions. Yet most inhalers depend on a single delivery pathway—oral or nasal. If that pathway is blocked, damaged, or misused, treatment fails.

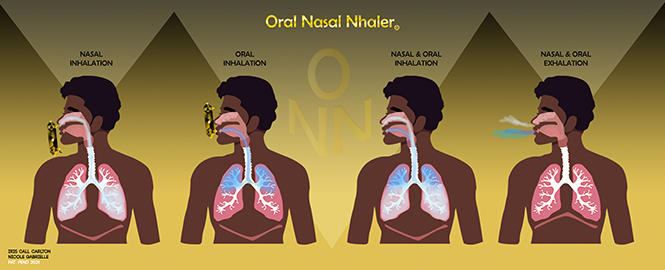
ONN introduces a hybrid inhaler system that delivers medication through both oral and nasal ports within one device. This dual-pathway design provides built-in redundancy, flexibility in administration, and improved reliability when patients need it most.
Dual oral and nasal delivery in one device
Two medication canisters (same or different therapies)
Twin dosage monitoring windows
Rescue and routine treatment in a single system
Designed to eliminate single points of failure
Reduces device burden, improves confidence during respiratory distress, and supports consistent medication delivery across age groups.
Supports multiple therapies, delivery preferences, and treatment scenarios without requiring separate devices.
Fewer devices, fewer failures, and simplified logistics for schools, hospitals, emergency kits, and home care.
ONN is designed as a modular inhaler platform capable of supporting multiple product lines, patient demographics, and therapeutic applications.
From adult and pediatric inhalers to portable and stationary units, ONN’s architecture enables rapid expansion across pharmaceutical, over-the-counter, and alternative inhalant markets.
Single delivery pathway
One medication per device
No redundancy
Separate rescue and maintenance inhalers
Failure equals no treatment
Dual oral and nasal delivery
Two medications in one device
Built-in fail-safe
Rescue and routine combined
Designed to reduce failure risk
ONN is protected by a robust intellectual property portfolio that includes over 150 domestic and international patents, design protections, trademarks, and a proprietary respiratory breathing technique.
Extensive global patent coverage
Dual-pathway inhaler protection
FDA 510(k) premarket notification pathway
Early FDA feedback recognizing ONN as a “game-changer”
Direct and partner-based inhaler sales
Pharmaceutical and global licensing opportunities
Strategic partnerships and in-house production roadmap
ONN Respiratory Technique and educational content
ONN is seeking $100 million in funding and a strategic equity partner to support manufacturing, FDA clearance, global licensing, and market expansion.
Ideal partners bring expertise in manufacturing, distribution, research and development, and global commercialization.
ONN’s mission is to redefine respiratory care by delivering safer, more reliable inhalation therapy for patients of all ages. By eliminating single points of failure and building a scalable inhaler platform, ONN is positioned to become the next standard in respiratory medical devices.
